Anaphylaxis is a life-threatening allergic reaction of rapid onset that can affect people of any age, in any setting.1,2 First-line management – intramuscular adrenaline – is universally agreed to be life-saving,1 and provision of AAIs to patients following an initial reaction should ensure prompt treatment in the event of recurrence. Despite the clear consensus on treatment for this life-threatening condition, substantial gaps exist in practice.
Evidence reveals areas for improvement in the management of anaphylaxis and AAI use in all settings,1,3 across healthcare professionals, patients and caregivers alike.3 The focus of management is all too often placed on the initial episode, with long-term management of at-risk patients potentially being overlooked.3
A retrospective survey of first aid anaphylaxis management in children prescribed an AAI reported that it was used in less than a third of anaphylactic reactions – just 29%.4 Potential barriers to administration include reluctance to carry the device, fear of needles and uncertainty about when and/or how to administer the AAI.3,5,6
Why do these gaps in practice persist today in the UK, and how can the pharmacist address them?
Anaphylaxis and the role of AAIs
While the Resuscitation Council acknowledges that there is no universally agreed definition of anaphylaxis, they characterise it as a ‘severe, life-threatening, generalised or systemic hypersensitivity reaction’.
A broad range of anaphylactic triggers (allergens) exist, with food, drugs and venom being the most commonly identified.7 Anaphylaxis is the likely diagnosis if exposure to a trigger is associated with rapid onset of skin changes (within minutes of exposure) and life-threatening compromise of the airway, breathing or circulation.7
Approximately 20 deaths from anaphylaxis are reported annually in the UK, although this may well be an underestimate.7 The incidence of anaphylaxis has increased over the past decade or two in many parts of the world, including the UK.8 The risk of recurrent anaphylactic reaction following an initial reaction is high, estimated at approximately 1 in 12 per year.7
As described above, immediate intramuscular injection of adrenaline constitutes first-line treatment.1 Adrenaline is the only medication that reduces hospitalisation and death in anaphylaxis. Alpha-1 agonism prevents and relieves airway oedema, hypotension and shock, while beta-1 agonism strengthens cardiac contraction and brings about bronchodilation.2,9 Delayed injection of adrenaline is associated with increased risk of fatality.1
Resolution of the acute episode, of course, does not signal the end of treatment of anaphylaxis.9 Given the unpredictable nature and rapid onset of anaphylaxis, guidelines recommend the provision of AAIs for patients with a view to rapid treatment of future anaphylactic episodes.1,2 Prescription of an AAI must be combined with specialist advice on allergen avoidance, a written treatment plan and clear training in AAI use.10
Potential pitfalls in AAI provision and use
• AAI availability
Patients prescribed AAI devices should make sure that they are accessible at all times, since they can’t predict when an anaphylactic episode will occur.11 And yet an alarming number of patients fail to do so; the proportion who do not keep their autoinjectors with them ranges, in different studies, from as low as 15% to 75%.1,2,5,11
Young people may feel uncomfortable carrying the device due to social implications, or may simply forget to do so. Pharmacists are well placed in the community to emphasise the importance of keeping AAIs to hand at all times. Fear of using the device, a considerable obstacle for many patients, must also be addressed during consultation.5
• Administration technique
As well as carrying the AAI device, it is imperative that patients or their parents/carers know how to use it properly; the technique varies depending on the device.10,12 Surprisingly frequently, patients, carers and healthcare professionals alike are uncertain about the correct method for AAI use.2,3,11
Worryingly, one study suggested that only 30–40% of patients were able to correctly demonstrate how they would self-administer adrenaline.2
There is a clear need to improve education on AAI use.2 Training in both when and how to use the AAI should be provided at the time of prescribing, and the pharmacist is ideally positioned to reinforce this training when dispensing the device.
Pharmacists can also encourage patients and parents/carers to obtain and use trainer pens (free from company websites), as well as accessing other support materials from company websites and videos such as those of the Anaphylaxis Campaign.10
• Expiry dates and storage
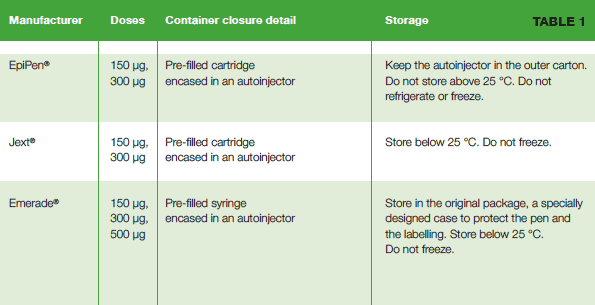
AAIs have a fixed shelf life, and it is important that patients are aware of the upcoming expiry dates for the devices that they carry.2,10,11 This can be difficult; given that the need to use the device tends to be both unpredictable and infrequent, the importance of checking medication and its expiry date can be forgotten.11 The Emerade, EpiPen and Jext devices all allow patients to register for alerts as to when the AAI needs replacing.10 Similarly, it is important that patients are aware of how to properly store their AAIs (see table 1).
• Dosing
The lowest AAI dose, 150 μg, is recommended for infants from the age of 6 months, as the cut-off for this strength is a minimum weight of 15 kg and avoidance should be possible for infants below 6 months of age. The AAI dose of 300 μg is recommended for children over 30 kg in weight, and for adults.10
One study indicated that some children were incorrectly prescribed a low-dose autoinjector even though they reached a weight of 30 kg, which would require a full dose.3,13 Children should be weighed regularly to ensure they are not on an incorrect dose.
• Number of devices
The MHRA recommends that two AAIs are prescribed for patients at risk of anaphylaxis, and that these patients carry both AAI devices at all times. This is due to uncertainties about the site of drug delivery and the speed of adrenaline action within the body.14 A UK study of AAI use in children and teenagers found that almost a third (32%) who used their AAI during anaphylaxis required more than a single dose.15
Available AAI devices
AAI devices utilise a multi-step technique that differs from one device to another. Instructions are provided in the package inserts, and manufacturers offer varying degrees of informational materials and support.
The two main types of AAI delivery systems are cartridge-based or syringe delivery.11 Cartridge-based delivery appears to offer some advantage over syringe delivery,11,16 as adrenaline release only occurs once the needle is fully extended into the tissue. Syringe delivery carries a risk of adrenaline deposition throughout the needle track, potentially reducing the medication that reaches target muscle.11
An overview of available AAIs is provided in table 1 below.17-24
References
1. Song TT et al. Allergy 2014;69:983–991
2. McLeane-Tooke A et al. BMJ 2003;327:1332–1335
3. Kastner M et al. Allergy 2010;65:435–444
4. Gold MS and Sainsbury R. J Allergy Clin Immunol 2000;106:171–176
5. Lange L. Allergo J Int 2014;23:252–60
6. Gallagher M et al. Clin Exp Allergy 2011;41:869–877
7. Resuscitation Council UK. Emergency treatment of anaphylactic reactions. Guidelines for healthcare providers. January 2008
8. Turner PJ and Campbell DE. Curr Opin Allergy Clin Immunol 2016;16:441–50
9. Simons FER et al. World Allergy Organ J 2015;8:32
10.Ewan P et al. Clinical & Experimental Allergy 2016;46:1258–1280
11.Frew AJ. Allergy 2011;66:15–24
12.MHRA. Adrenaline auto-injectors: updated advice after European review. August 2017
13.Blyth TP and Sundrum R. Arch Dis Child 2002;86:26–27
14.MHRA. Drug Safety Update. Volume 11, Issue 1. August 2017
15.Noimark L et al. Clin Exp Allergy 2011;42:284–292
16.Schwirtz A and Seeger H. J Asthma Allergy 2010;3:159–167
17.MHRA. Adrenaline auto-injectors: a review of clinical and quality considerations. June 2014
18.EpiPen Adrenaline (Epinephrine) Auto-Injector 0.3 mg. Summary of Product Characteristics. March 2018
19.EpiPen Jr Adrenaline (Epinephrine) Auto-Injector 0.15 mg. Summary of Product Characteristics. March 2018
20.Emerade, 500 micrograms, solution for injection in pre-filled pen. Summary of Product Characteristics. September 2016
21.Emerade, 300 micrograms, solution for injection in pre-filled pen. Summary of Product Characteristics. September 2016
22.Emerade, 150 micrograms, solution for injection in pre-filled pen. Summary of Product Characteristics. September 2016
23.Jext 300 micrograms Solution for Injection in prefilled pen. Summary of Product Characteristics. June 2018
24.Jext 150 micrograms Solution for Injection in prefilled pen. Summary of Product Characteristics. June 2018